Organizations who perform bronchoscopy using Olympus brand bronchofiberscopes and bronchovideoscopes are advised to review this product's corrective action. On October 12, 2023, Olympus sent all affected customers an Urgent Medical Device Corrective Action—notifying its customers of adverse event complaints and reminding users of the manufacturer’s warnings related to the use of high-frequency therapy equipment. This notice was generated due to risks of burns and fire in certain conditions. Note that this recall is a correction for how these products should be used and not a product removal.
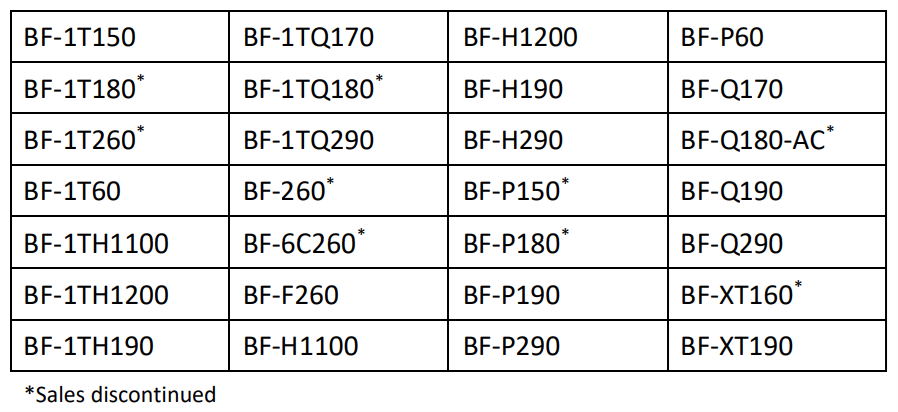
The affected scope models are:
Organizations are advised to review and follow the Urgent Medical Device Corrective Action notice above for specific actions.
Please contact Courtemanche and Associates for questions at 704-573-4535 or info@courtemanche-assocs.com.