Survey Readiness – Top 12 Survey Readiness Tips for 2022

Our team’s Top 12 Survey Readiness Tips for 2022. This list is based upon common, problematic findings from our accreditation and regulatory consulting travels across the country in 2021.

Our team’s Top 12 Survey Readiness Tips for 2022. This list is based upon common, problematic findings from our accreditation and regulatory consulting travels across the country in 2021.

Throughout our travels across the country, we encounter organizations that struggle with the management of their data. We believe that CMS and the accrediting agency, The Joint Commission, may also be seeing the same thing.
On September 1, 2021, The Joint Commission posted the long-awaited Proposed Changes to the Emergency Management Chapter.
On August 4, 2021, The Joint Commission posted new elements of performance related to MM 09.01.01 Antibiotic Stewardship.

Titrating medications is the process of adjusting medication dosage for maximum benefit without adverse side effects. It can be a fine line to walk
Many organizations may be leaving these opportunities for improvement on the table. Did you know that any toothpaste that contains sodium fluoride contains a National Drug Code and is classified as a medication? We recently verified this with The Joint Commission Standards Interpretation Group. Their team advised this would be determined by how they are […]
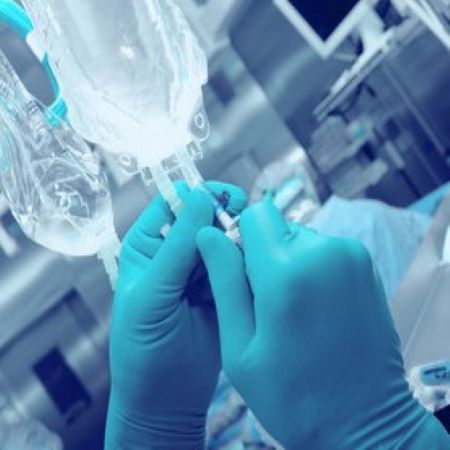
Titrating intravenous medications is the process of adjusting medication dosage for maximum benefit without inducing adverse side effects. It can be a fine line to walk, but when staff follow clearly defined parameters through well-crafted titration orders, it can be performed with a high degree of safety and ensure that your practice is aligned with […]

How often do we go to the linen closet and say to ourselves, “is this linen safe to put on my patient’s bed?” As a nurse for over 40 years, I do not think that I have ever asked myself that question. I was happy that the linen was there and that I could use […]
1. Competency: With advances in technology and more complex responsibilities amongst staff, the area of competency assessment and reassessment holds a lot of opportunity for improvement. Many organizations struggle with differentiating orientation and competency at the point of hire along with determining which competency assessment should occur on an annual basis. 2. Suicide Risk Identification […]
Do you ever wonder what a mock survey consultant does prior to visiting your organization? Have you been curious about the extent of preparation that occurs, how we are able to identify areas for improvement (or regulatory gaps) in a short period of time, and how it all comes together in a final report to enable effective resolution?